What Type of Reaction Is Occurring Between I2 and Zn
A Reaction where a metal replaces a cation in an ionic compound or acid. Zn I 2 ZnI 2.

Aamc Fl 3 C P Question 16 Logic Check R Mcat
When solutions of two ionic compounds are combined and a solid forms the process is called.

. ZnC2H3O22 aq Na3PO4 aq NaC2H3O2 aq Zn3PO42 s Question. What type of reaction is occurring between i2 and zn. State your solution to the problem is the redox reaction as given spontaneous or not.
See answer 1 Best Answer. In this video we determine the type of chemical reaction for the equation Zn HCl ZnCl2 H2 Zinc Hydrochloric acid. Since we have a metal replacing a.
It is also called a substitution reaction. At the cathode Zn2 ions are reduced to form Zns. What is the molarity of a 250.
But this reaction is not as simple as its equation looks. The process in which electrical energy is used to drive a nonspontaneous electrochemical reaction is called. Enter an equation of a chemical reaction and click Balance.
Synthesis and combustion. Fe Au Co Br C O N F. Synthesis decomposition single replacement double replacement.
This reaction takes place at a temperature near 60C in the water. The half reaction for the oxidation reaction omitting phase labels is as follows. 0 m stock solution.
Iodine vapor is produced when water is added. Zn I 2 ZnI 2. Ml h2so4 solution that was made from a 20.
What is the period of the voltage source that operates the plasma pencil. 0 m stock solution. 1000ns Because the period of a time-varying signal is the shortest repitition time.
The answer will appear below. This is most easily demonstrated with fluorine chlorine bromine and iodine. Balance the following equations and determine which type of reaction is occurring.
What type of reaction is occurring between I2 and Zn. Thus you have to understand the activity series. Always use the upper case for the first character in the element name and the lower case for the second character.
The reaction is acombination reaction and also a redox reaction. I believe its not precipitate formation because precipitate formation is supposed to be exothermic and generally spontaneous. If they had to add methanol and heat the reaction to get the final product then the reaction is endothermic and.
0 ml of a 10. What type of reaction is occurring between i2 and zn. Which type of reaction might occur.
A chemical reaction in which ions gets exchanged between two reactants which form a new compound is called a double displacement reaction. What is the molarity of a 250. Consider the partial equation.
Cl2 2RbI 2RbCl I2 I2 NiBr2 NiI2 Br2. ZnI 2 is formed. It takes the form of XY ZA XZ YA.
Its necessary to know the seven elements that occur in nature as diatomic molecules in order to construct and balance the equation correctly H2 N2 O2 F2 Cl2 Br2 and I2. Ml h2so4 solution that was made from a 20. The experiment can be extended to show the decomposition of a compound into its elements.
If Zn metal and I2 are mixed no reaction takes place. I was between redox and precipitate formation. 0 ml of a 10.
The half-reaction occurring at the anode is represented by 2I-l I2s 2e-. Zinc react with iodine to produce zinc iodide. Zn CuSO4 Cu ZnSO4 2Na2O 4Na O2.
Introduction Introduction Chemical reactions occur when bonds between the outermost parts of atoms are formed or broken Chemical reactions involve changes in matter the making of new materials with new properties and energy changes. Zns I2s ZnI2s and will go to completion after a period of several minutes. Mixture of Zn I 2 before water is added.
Collectively these elements are called the halogens and are in the next-to-last column on the periodic table see Figure 41 Halogens on the Periodic Table. The interaction of an element with oxygen to create an oxide is a common example of a combination reaction. Zn CuSO 4 ZnSO 4 Cu.
Zn Zn 2 2e. When solutions of two ionic compounds are combined and a solid forms the process is called. Balance water as a separate H and OH 108 Single Replacement Reactions M AX MX A Single Replacement.
An exothermic redox reaction occurs forming zinc iodide which can be obtained by evaporating the solvent. Example of displacement reaction. The reaction of zinc metal with iodine shows direct combination decomposition recrystallization of sublimed I2 and electrolysis.
It is also called a metathesis reaction. Co - cobalt and CO - carbon monoxide. Zinc and Iodide combine to form zinc iodide.
If a reaction will occur write a balanced net ionic equation for the reaction assuming that the product ions are. This half reaction is balanced in terms of the number of zinc atoms and it also shows the two electrons that are needed as products to account for the zinc atom losing two negative charges to become a 2 ion. This experiment can be used to illustrate the differences between metallic and non-metallic elements and their reaction to form a compound a metal salt.
Zinc atoms are oxidized to Zn 2. The metal has to be more active than the cation for the reaction to occur. A combinationreaction is when two elements combine.
This question tripped me up too. Filter contents to isolate clear colorless ZnI 2. Balance acid base reactions Tip.
To enter an electron into a chemical equation use - or e. The reaction between zinc and iodine to produce the ionic compound zinc iodide is. Use a table of Standard Reduction Potentials to predict if a reaction will occur between Zn metal and I2s when the two are brought in contact via standard half-cells in a voltaic cell.
If no reaction will occur leave. And this reaction is the same as the reaction we were given that is we were given a piece of magnesium metal Mg s to place in an aqueous solution of copperII ions Cu 2 aq so we expect a reaction between these two substances to occur spontaneously. Oxidation-reduction because both Zn and I2 change oxidation states during the reaction 18.
Not all proposed single-replacement reactions will occur between two given reactants. A chemical reaction is defined as the process by which the atoms of one or more substances are rearranged to form a different. SPOILER AAMC FL3 CP 16.

Electrochemistry Electrochemistry Chemistry Experiments Science Projects For Kids

Electrochemistry Electrochemistry Science Electricity Electronic Engineering

Type Of Reaction For Zn Cuso4 Znso4 Cu Youtube
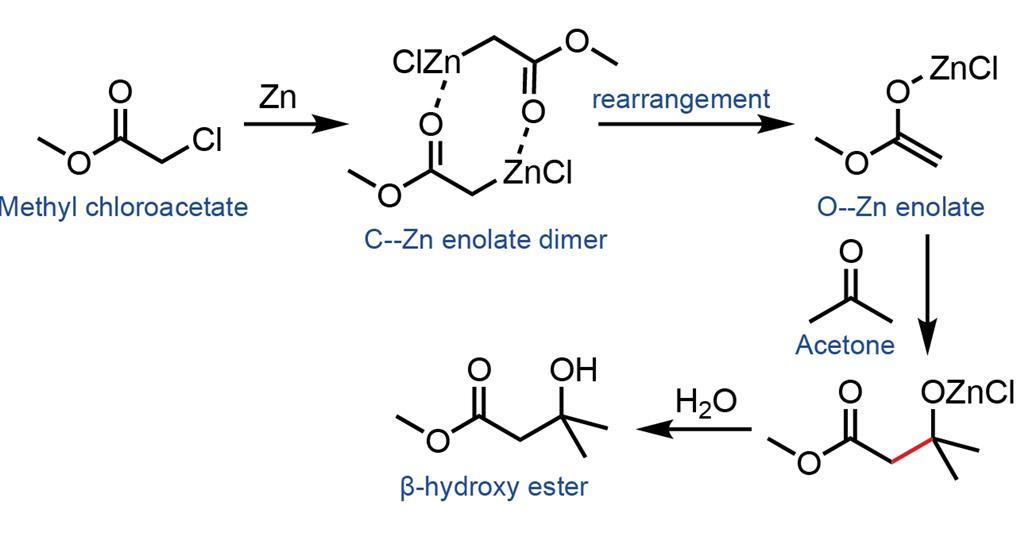
Reformatskii Reaction Opinion Chemistry World
The Following Cell Is Set Up Zn S Zn2 Aq Cu2 Aq Cu S Write Down The Equation For The Reaction Which Takes Place In Each Half Cell From Which Electrode Do Electrons Flow Away From And Into The

Type Of Reaction For Zn O2 Zno Youtube

Solved Answer The Questions Using This Reaction Zn S Cuso4 Aq Znso4 Aq Culs What Is The Oxidation State Of Zinc Zn On The Reactant Side What Is The Oxidation State Of Zn On
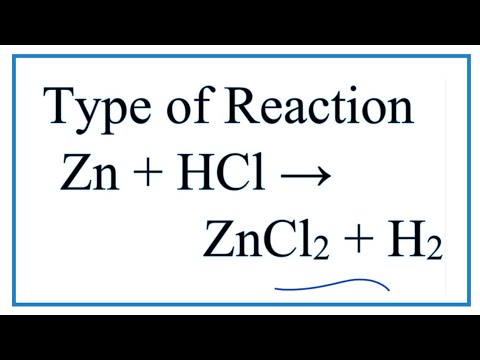
Type Of Reaction For Zn Hcl Zncl2 H2 Youtube

Periodictableofelements Periodictable Bromine Chemistry 101 Science Education Chemistry

How To Write The Net Ionic Equation For Zn Ch3cooh Ch3coo 2zn H2 Youtube
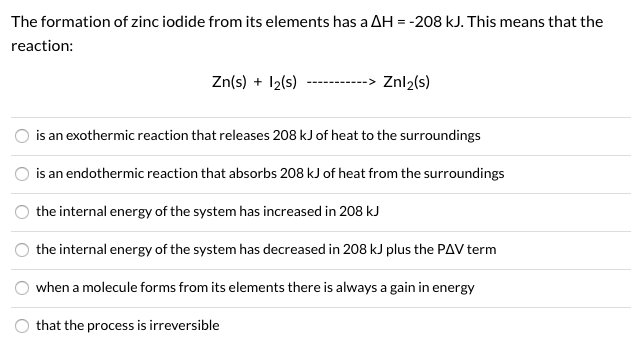
Solved The Formation Of Zinc Iodide From Its Elements Has A Chegg Com

The Chemistry Nobel Prize Ensenanza De Quimica Profesor De Quimica Lecciones De Quimica
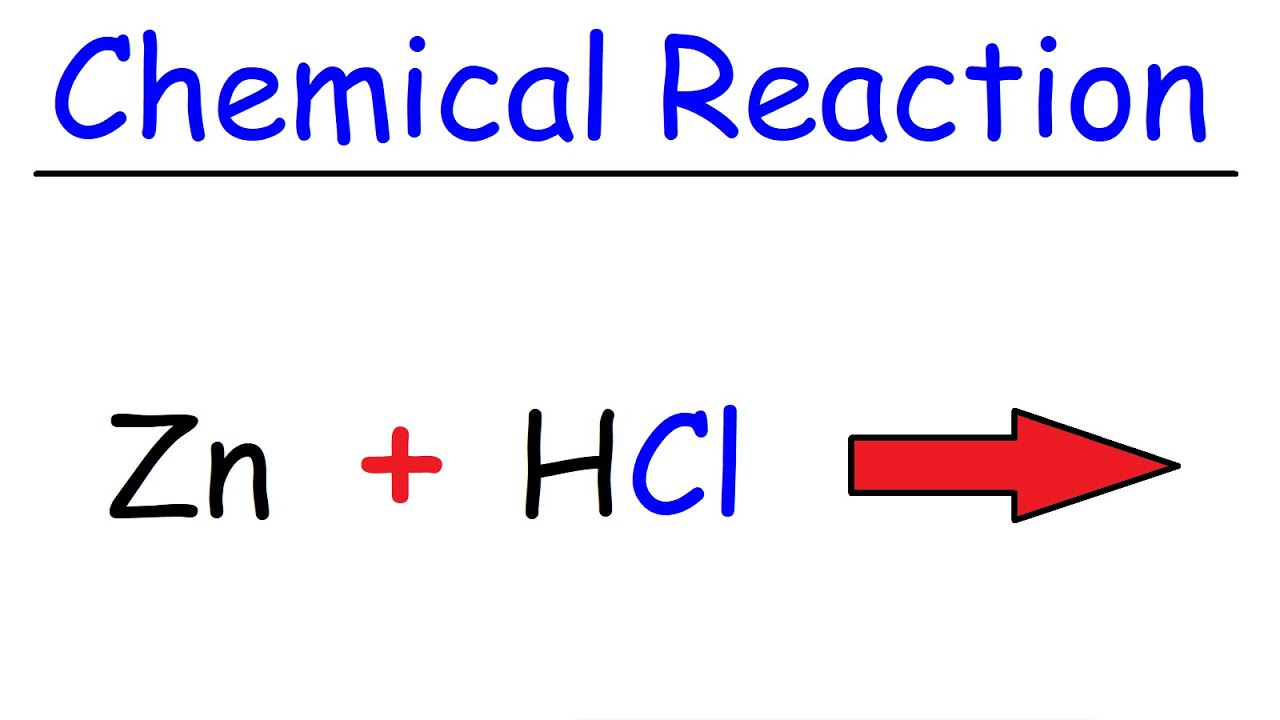
Zn Hcl Reaction Zinc Hydrochloric Acid Youtube
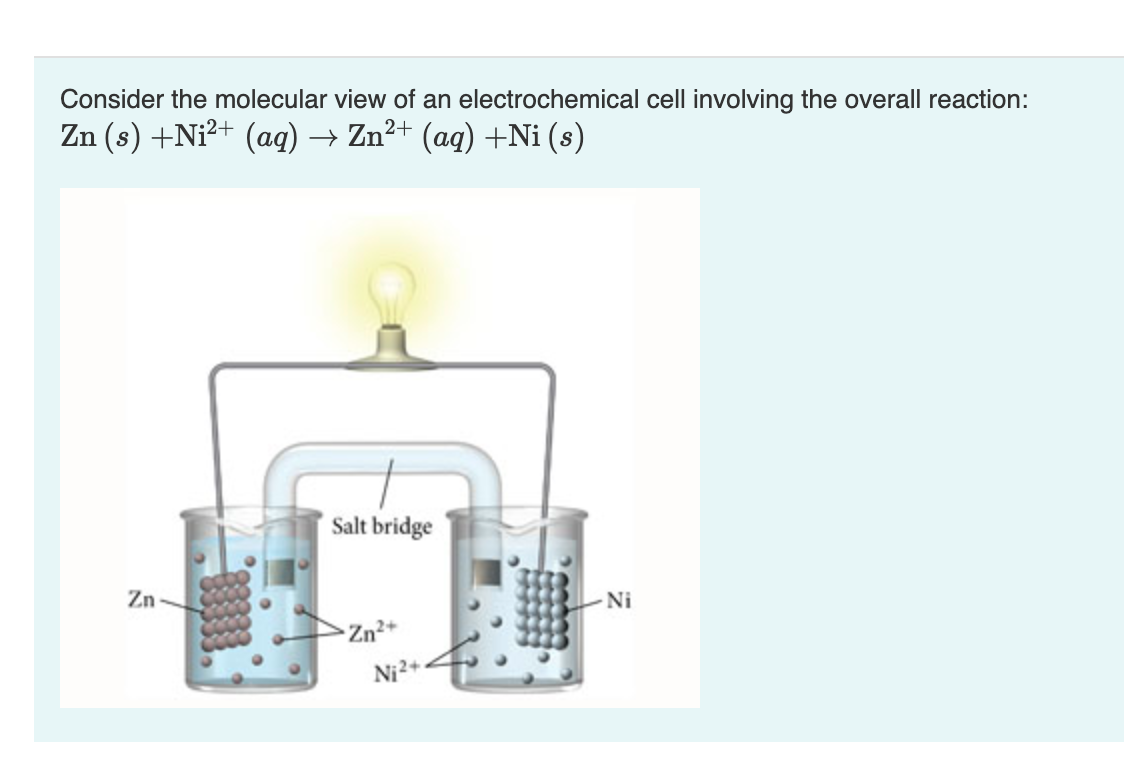
Solved Consider The Molecular View Of An Electrochemical Chegg Com

Chemistry Experiment Reaction Between Iodine And Zinc Chemistry Experiments Cool Chemistry Experiments Organic Chemistry Study




Comments
Post a Comment